European Commission DG for Internal Market, Industry, Entrepreneurship and SMEs Unit D4 – Health Technology & Cosmetics
UDI book "Unique Device Identification"
Basics, practical solutions, answers for manufacturers, clinics and users of medical devices and in-vitro diagnostics
The 257-page UDI book by author Heinrich Oehlmann describes the implementation of UDI in accordance with the regulations for labelling and data registration for medical devices and in-vitro diagnostics. It contains descriptions of the accredited barcode labelling systems with practical tips for selection of most suitable codes and the criteria for registration in the officially managed databases in the USA and Europe. In addition, the obligations of manufacturers, importers, distributors and hospitals from the legal texts for medical devices (MDR) and IVD products (IvDR) are interpreted. Implementation guidance includes the general use of linear barcode, 2D codes and RFID for automatic data capture and how to derive practical benefit from the obligations.
This information is intended for manufacturers as well as for hospital management and it’s quality and IT officers.
The UDI Book II is available in print and as an e-book in German language:
ISBN 978-3-410-29843-4, ISBN (e-book) 978-3-410-29844-1
A reading sample can be accessed here:
https://www.beuth.de/de/publikation/udi-unique-device-identification/320019099#leseprobe

The UDI directive requires transmission into and management of UDI related data into GUDID (Global Unique Device Identification Data Base), the FDA's global data base.
GUDID serves the registration of medical devices and related manufacturers' data. Any device registered can be accessed by the general public, as well as everybody concerned professionally. As a future option, GUDID is to be linked to other data bases around the world, strongly promoting global recognition.
Based on universal standards for medical device identification, the data base comprises a key set of identifying elements for each possible UDI coding.
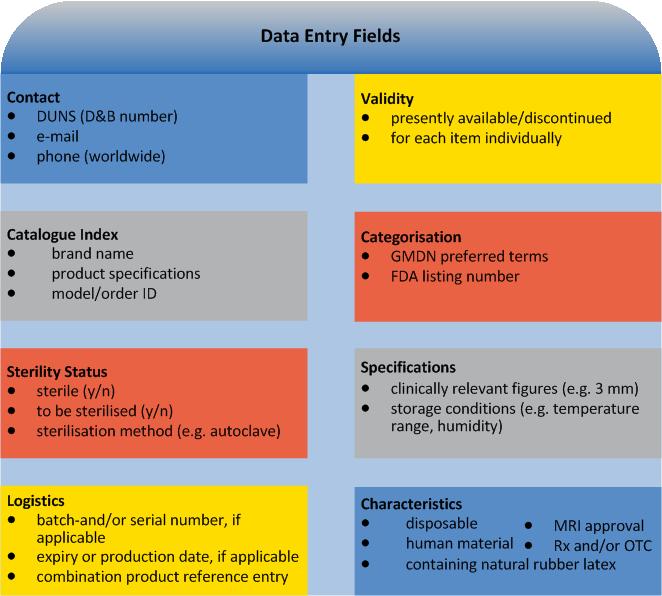
For each Device Identifier (DI) (no PIs): Trade mark proprietor, trade/brand name of the device. And furthermore:
applicable for new versions/models only: previous DI
type/model Number
applicable for directly marked devices only: DI other than indicated on type plate
dimensions of type/model
type of PI on type plate
FDA premarket submission and listing number(s)
Global Medical Device Nomenclature (GMDN) term
FDA product code (procode)
the number of individual devices in each package
commercial distribution status
higher levels of packaging
indication whether it is a kit, combination product or HCT/P
Furthermore UDI provides information on the individual device marking it:
as sterile or to be sterilised before use (nature and extent of sterilisation), advanced levels of packaging
as containing natural rubber latex
in respect of MRI compatibility (safe, conditional, unsafe)
as Rx and/or OTC
Currently more than 2,000 companies apply UDI-accredited HIBC codes. Among members, UDI can already be implemented quickly and without any difficulty (see: UDI for HIBC members). Manufactures, willing to utilise HIBC in the future, have to take three steps only. On the following pages you will find all relevant information, needed to label your products UDI-compliant.
To those, using HIBC codes already, UDI-compliant product labelling will be a simple task. Authorities in their implementation of UDI, refer to common practice. Referring to the good practice approach, there is no such thing as a new UDI code. First of all there is „ISO/IEC 15459 Unique Identification“, comprising registration rules for accreditation offices of singular company codes. Accordingly singularity of all UDI-DI is ensured by means of the preset sequence <accreditation office><labeller's code><product/device>.
Current HIBC members have to undergo an audit, following a quality inspection, ensuring UDI-DI and UDI-PI are applied correctly to the appropriate level of packaging.
Data transfer onto the central GUDID data base via the „HL7 SPL“-form is new, however. Doing so, all data entries, with the exception of the DUNS-Code or the GMDN classification code, which may be new to some manufacturers, rely on data already available. Nevertheless, these are open source codes; DUNS numbers are free of charge.
 HIBC-labelled products and devices are easily marked with the UDI-Code. UDI-compliant labels will automatically be clearly equipped with the HIBC badge showing, the manufacturer is well prepared for global developments, at a glance. The HIBC badge is proof for UDI compliance!
HIBC-labelled products and devices are easily marked with the UDI-Code. UDI-compliant labels will automatically be clearly equipped with the HIBC badge showing, the manufacturer is well prepared for global developments, at a glance. The HIBC badge is proof for UDI compliance!
The HIBC Healthcare Barcode is a unique global product identifier, comprising extended product data in a concise data structure. Developed back in 1986 to ensure high-security track and trace, HIBC is recognised for one-of-a-kind security until present.
HIBC is a widely recommended global application within the ISO 22742 Standard for labelling product packaging. The standardised HIBC structure is compliant with all alphanumeric ISO-Symbologies, as well as with RFID (Radio Frequency Identification). Manufacturer- and product codes (REF), consisting of up to 18 alphanumeric digits, comprising entry fields for quantity, LOT, serial number, expiry and manufacturing dates, are supported. The extended form includes add-on information, e.g. integrated hyperlinks (URL).
REF in combination with a manufacturer ID, serve as unique global reference for scanning and entries into the UDI data base. HIBC contains all information in a transparent manner. Secondary encoding to identify manufacturer and item number is not necessary.
HIBC is a unique product code, containing planned and actual data over the product's entire life cycle.
HIDC is fully UDI accredited by the FDA (Food and Drug Administration), as well as by the IMDRF (International Medical Device Regulatory Forum).
The REF number is directly included into the HIBC code.
HIBC innovatively utilises identical code symbols on packaging and device.
HIBC symbologies are adequate for labelling smallest devices.
HIBC is fully compliant with other coding systems.
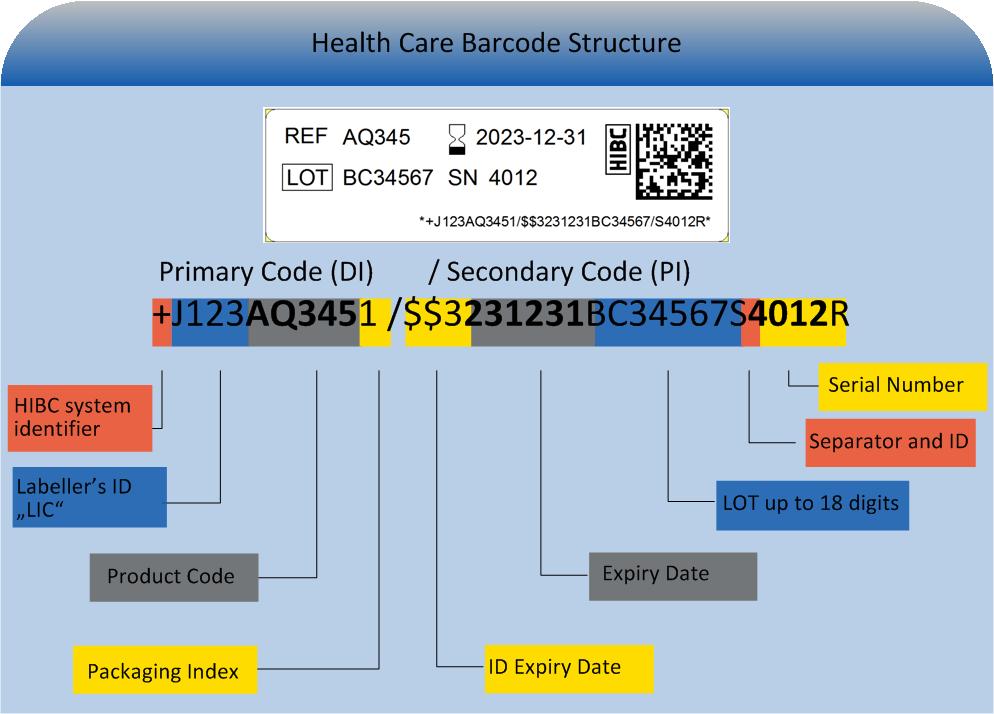
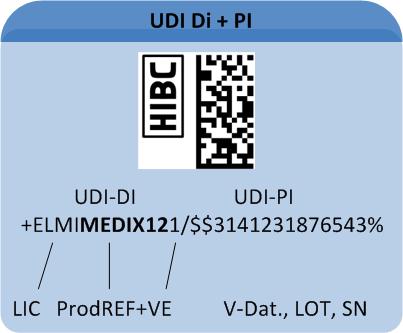
The HIBC code is composed of the primary code (UDI"DI"), containing the product reference, a "/" as delimiter symbol, the secondary code (UDI "PI") containing production data and a terminatory Modulo 43-compliant check character.
Primary code (UDI-DI)
The primary code (UDI"DI") builds a unique product reference incorporating these data fields:
Secondary code (UDI-PI)
The secondary code (UDI-PI), containing production data is designed variably. It may contain the following entries:
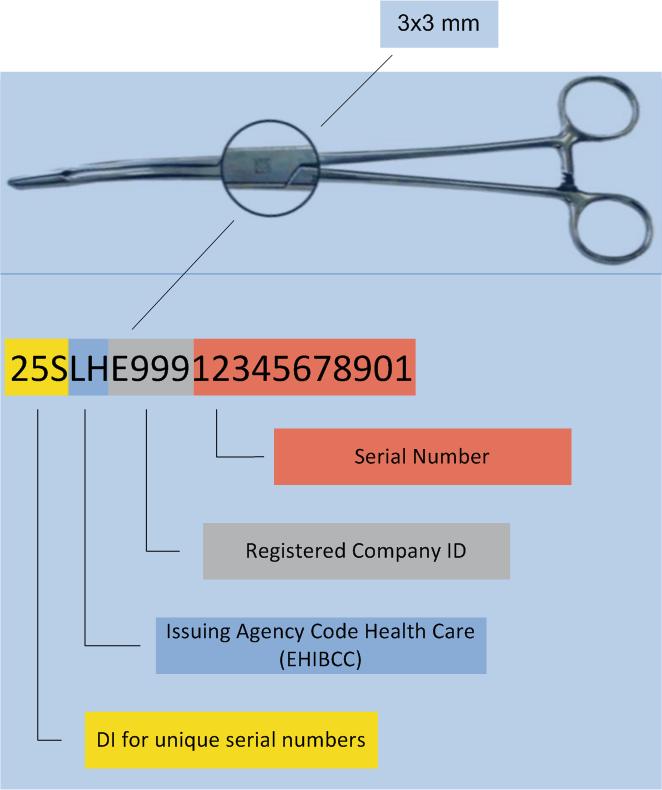
Legal provisions require direct labelling of reprocessed medical devices (e.g. surgical instruments), as well as implants, requiring sterilisation.
To put small surface labelling to work, HIBC utilises the „Unique Identification Mark“ (UIM) data structure.
UDI is an acronym for "Unique Device Identification". It describes a global standard of product labelling for the medical sector. UDI comprises unique labelling of manufacturer- and product related information on the product itself or its packaging, as well as manufacturer-related master data entries in a central data base.
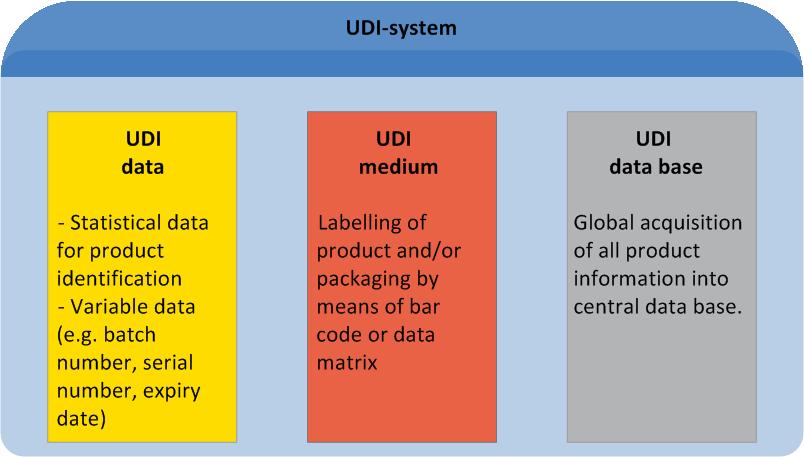
Manufacturers and users alike, will benefit from UDI at every single stage of the distribution chain (for details see "UDI Benefits").
Manufacturers will utilise the unambiguous code for faultless and prompt tracking throughout the production-, stocking-, picking- and shipment process.
In logistics, the unique barcode has a proven track record. UDI allows for tracking of positions, transport and process management (e.g. sterilisation) alike. Hence manual entries will be avoided, while processes will be fully automated and faultlessly well-documented.
Consignees benefit from UDI all the way from receipt of goods, interim storage through restocking. Beyond consideration is the fact, that UDI enhances patients' safety by making medical devices traceable at any point.
Until 2020, the implementation of UDI labelling will be mandatory, to be completed in phases between 2014 and 2020, according to risk factors of the individual product. From a certain appointed date, medical products must solely be supplied with appropriate UDI encoding.
Class III devices must fully comply with the directive from September 2014. Until September 2015 all Implants with palliative or life supporting function must comply with the UDI directive.
Class II medical devices must comply with the directive from September 2016, Class I devices from September 2018.
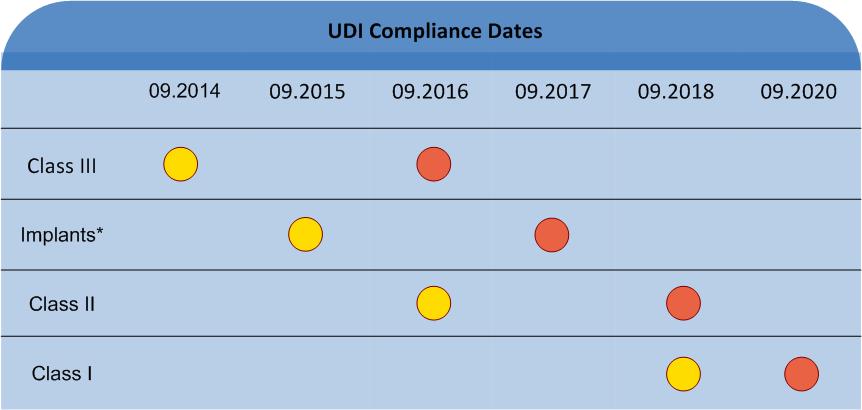
* Class II and I implants; palliative or life supporting medical devices
Mandatory UDI labelling on packaging
Mandatory UDI labelling on product/device
Apart from recent regulations in the US and Turkey, regulations are under way for the EU and Asia. At the pan-European level negotiations hint at a globally interoperable level of regulation. Manufacturers exporting to countries, other than the US or Turkey, should immediately shift to UDI-compliant codes, in order to be prepared for upcoming developments.
A UDI-compliant Code comprises all relevant data for appropriate device traceability. The UDI Code comprises two components (for details on code structure see "Healthcare Barcode"):
1: The "UDI Device Identifier" (UDI-DI) includes the manufacturer's/labeller's ID, the product's reference code and – optional – a code on its packaging.
2: The variable "UDI Production Identifier" (UDI-PI) may include batch number, serial number or production date.
Once completely implemented, manufacturers, suppliers, health facilities and patients will benefit from UDI.
UDI will found a basis for a secure, global distribution chain, prepared for
emergencies, whilst avoiding aberrancy and product piracy.
Manufacturers benefit from UDI by means of a rapid detection throughout
the production, stocking, order-picking and handling/shipping process.
Consignees benefit from UDI with the detection of incoming goods, interim
storage and restocking.
UDI facilitates logistics, encompassing location, logistic movements and
handling in transport.
UDI allows for a more precise system of documentation and reporting. Blun-
der can be located and analysed at short notice.
UDI enables medical professionals and others to analyse individual devices
unmistakably and promptly, to obtain information concerning their specifics
and thus avoid medical mistake.
UDI provides manufacturers, distributors and health facilities with standardised
product identification, facilitating product recalls.
UDI leads the way towards a globally recognised identification and detection
method for medical technology. UDI creates a universal standard, fostering
technical analysis of all currently marketed medical technology. Thanks to UDI,
knowledge acquired can be implemented into clinical data processing, electronic
medical records and databases. Comprehensive market monitoring may provide
particulars for the necessity for research and development of medical technology
and it current sales figures, whilst allowing for conclusions concerning new
applications of technologies already available.
quote: FDA final rules 24-09-2013
Page 58786
Summary, sentences:
... and requires that each UDI be provided in a plain-text version and in a form that uses automatic identification and data capture (AIDC) technology.
§ 801.40 Form of a unique device identifier.
(a) Every unique device identifier (UDI) must meet the technical requirements of § 830.20 of this chapter.
The UDI must be presented in two forms:
(1) Easily readable plain-text, and
(2) Automatic identification and data capture (AIDC) technology.
(b) The UDI must include a deviceidentifier segment.
PLAIN TEXT refers to the UDI but not to other label content. This means that the full UDI data string shall be printed as Human Readable Interpretation (HRI) which represents the encoded UDI data string in a linear or 2-d bar code, e.g. +ELMIMED121/$$1216L1234R
Note: Other text fields printed on the label for Company, REF, LOT, SN and relevant symbols are subject to other regulations as well, as the Expiry Date printed as format YYYY-MM-DD but can be encoded as MMYY in the symbol (see also question 6).
quote: FDA final rules 24-09-2013
58791, Table 1) ...requires only that a label ‘‘disclose’’ the presence of AIDC technology. See § 801.40(c).
55802, U:) FDA has simplified this provision, now at
§ 801.40(c), so that it requires that the label or device package disclose the presence of AIDC technology without specifying how.
The HIBC Emblem fulfills the function of indicating that UDI is present in a code. FDA final rules require that it shall be seen that a UDI is present on a label or device package but FDA does not specify how. Therefore “+UDI” could be add to the HIBC Emblem, example:

Link for download of the emblems: www.hibcc.org
quote: FDA final rules 24-09-2013
58826, § 830.40
(b) A device identifier shall be used to identify only one version or model.
(c) In the event that a version or model of a device is discontinued, its device identifier may not be reassigned to another device.
§ 830.50 Changes that require use of a
new device identifier.
(a) Whenever you make a change to a device that is required to bear a unique device identifier (UDI) on its label, and the change results in a new version or model, you must assign a new device identifier to the new version or model.
(b) Whenever you create a new device package, you must assign a new device identifier to the new device package.
58787 A device identifier that corresponds to the specific version or model of the device
58789 LL. Information Required for Unique Device Identification—Information Concerning Each Version or Model of a Device—§ 830.310(b)
58794 The final rule defines version or model to mean ‘‘all devices that have specifications, performance, size, and composition, within limits set by the labeler.’’
58807 If the labeler makes a change to a device that is required to bear a UDI on its label, and determines that the change results in a new version or model, the labeler must assign a new device identifier to that device and to all associated device packages.
58817 Device package means a package that contains a fixed quantity of a particular version or model of a device.
58818 (1) A device identifier—a mandatory, fixed portion of a UDI that identifies the specific version or model of a device and the labeler of that device
Yes, each model revision requires a new UDI to be registered and labeled.
With HIBC the body of the HIBC Primary Code can stay where the labeler may add an alpha or numeric revision indicator for building the new UDI-DI.
(For differenciation of packaging sizes the HIBC Unit of Measure applies.)
Note: Using the GS1 system a new GTIN generated for each model / version.
quote: FDA final rules 24-09-2013:
§ 801.20 Label to bear a unique device identifier.
(a) In general. (1) The label of every medical device shall bear a unique device identifier (UDI) that meets the requirements of this subpart and part 830 of this chapter.
58816, table 6
Class I devices, and devices that have not been classified into class I, class II, or class III that are required to be labeled with a UDI, must a bear UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use.
58814 03, § 801.45, any device that has to be labeled with a UDI also has to bear a permanent marking providing the UDI on the device itself if the device is intended for more than one use and intended to be reprocessed before each use.
The text just refers to “devices” but does not mention parts building the device.
quote: FDA final rules 24-09-2013:
58787
A *production identifier that more precisely identifies the specific device by providing variable information, such as the lot or batch, the serial number, expiration date, the date of manufacture,…
*Note: production identifier = UDI-PI
The option will be included with the Revision ANSI HIBC2.4 expected for 2014-Q1. It will include options for LOT&SN+*Manufacture Date. *The format as printed text is YYYY-MM-DD, e.g. 2014–01–02, but the HIBC encoded format is DIYYYYMMDD, e.g. 16D20130202
quote: FDA final rules 24-09-2013:
D. Formatting of Dates Provided on Medical Device Labels—§ 801.18 Format for the text: YYYY–MM–DD to harmonize with the ISO 8601 ... must be presented as year-month-
day (for example, 2013–09–30). Format for AIDC: …comments suggested that the date format should not apply to data communicated by AIDC technologies (e.g., bar codes and radiofrequency identification (RFID). FDA agrees that we should not attempt to regulate how data is communicated by AIDC technologies, or the order in which specific information is communicated by AIDC.
The final rules differentiate between a date format printed in human readable text and the format of the associated encoded date. Where the printed format “must be” YYYY-MM-DD the same date can be determined by the code structure and encoded in bar code differently.
If the encoded format is MMYY then the printed date is completed by the last day of that month, e.g. 0913 will be printed as text 2013-09-30.
Note: HIBC supports the format YYMMDD among the others.