What does UDI stand for?
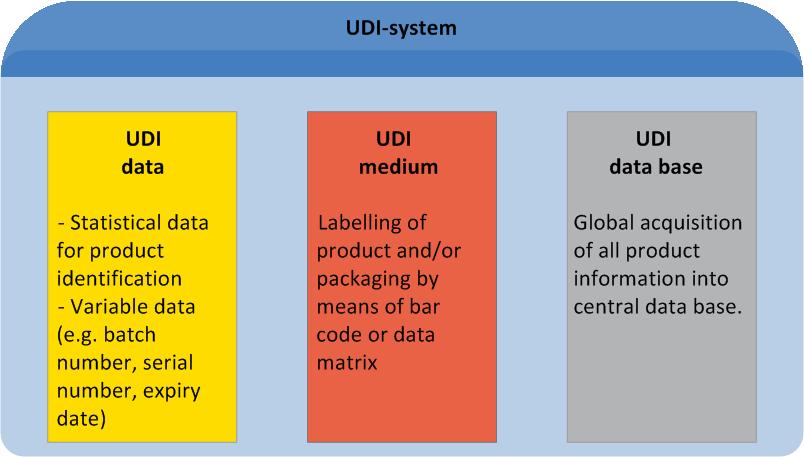
UDI is an acronym for "Unique Device Identification". It describes a global standard of product labelling for the medical sector. UDI comprises unique labelling of manufacturer- and product related information on the product itself or its packaging, as well as manufacturer-related master data entries in a central data base.
Why UDI?
Manufacturers and users alike, will benefit from UDI at every single stage of the distribution chain (for details see "UDI Benefits").
Manufacturers will utilise the unambiguous code for faultless and prompt tracking throughout the production-, stocking-, picking- and shipment process.
In logistics, the unique barcode has a proven track record. UDI allows for tracking of positions, transport and process management (e.g. sterilisation) alike. Hence manual entries will be avoided, while processes will be fully automated and faultlessly well-documented.
Consignees benefit from UDI all the way from receipt of goods, interim storage through restocking. Beyond consideration is the fact, that UDI enhances patients' safety by making medical devices traceable at any point.
When? UDI now!
Until 2020, the implementation of UDI labelling will be mandatory, to be completed in phases between 2014 and 2020, according to risk factors of the individual product. From a certain appointed date, medical products must solely be supplied with appropriate UDI encoding.
Class III devices must fully comply with the directive from September 2014. Until September 2015 all Implants with palliative or life supporting function must comply with the UDI directive.
Class II medical devices must comply with the directive from September 2016, Class I devices from September 2018.
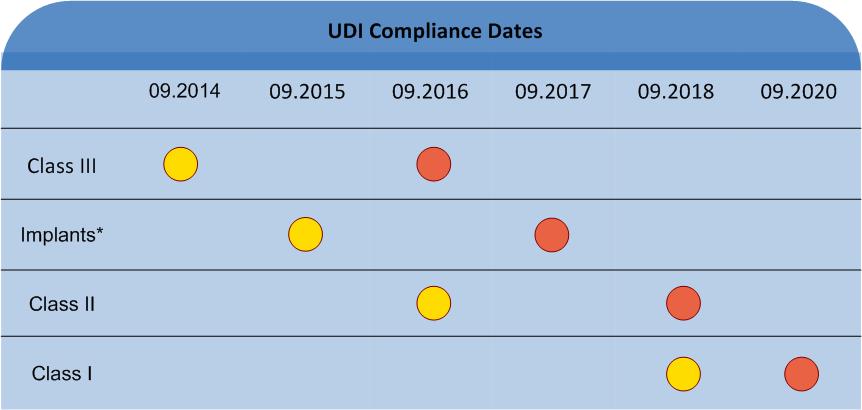
* Class II and I implants; palliative or life supporting medical devices
Mandatory UDI labelling on packaging
Mandatory UDI labelling on product/device
Where will UDI be introduced?
Apart from recent regulations in the US and Turkey, regulations are under way for the EU and Asia. At the pan-European level negotiations hint at a globally interoperable level of regulation. Manufacturers exporting to countries, other than the US or Turkey, should immediately shift to UDI-compliant codes, in order to be prepared for upcoming developments.
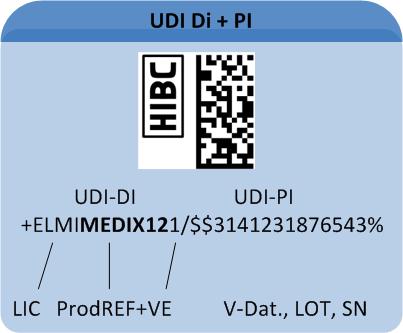
Which information does the code consist of?
A UDI-compliant Code comprises all relevant data for appropriate device traceability. The UDI Code comprises two components (for details on code structure see "Healthcare Barcode"):
1: The "UDI Device Identifier" (UDI-DI) includes the manufacturer's/labeller's ID, the product's reference code and – optional – a code on its packaging.
2: The variable "UDI Production Identifier" (UDI-PI) may include batch number, serial number or production date.